Which of the Following Statements Correctly Describe Molar Mass
There are 1600 amu of oxygen per molecule of acetic acid. Molar mass of CCl415381 gmol and molar mass of hexane8617 gmol 442 g 23.

Molar Mass Molecular Weight Of Na2so4 10h2o Youtube
Atomic mass of nitrogen 140g.
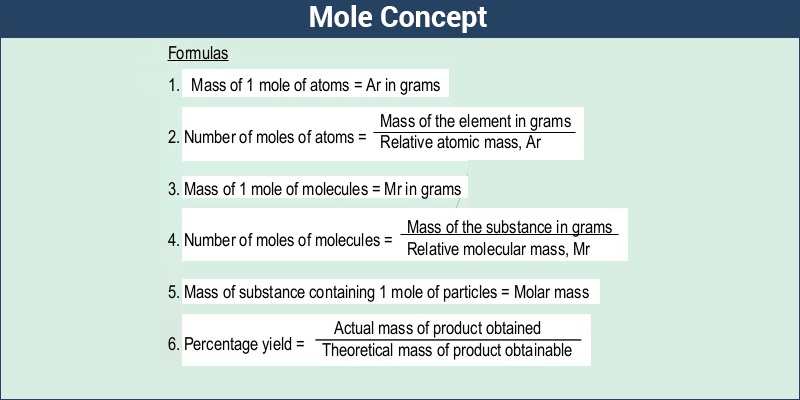
. Which of the following statements is NOT true. The term molar mass applies to compounds but not to elements. The molar mass of acetic acid is 6005 gmol.
Which of the options correctly reflect the steps needed to calculate the molarity M of the solution. The mass of oxygen atoms in a mole of acetic acid is 3200 g. Your answer is partially correct.
Density of the solution Mass to. Select all that apply. Heating or cooling during a phase change ----- q nΔH o change heating or cooling within a phase if mass is given ----- q msΔT A sample of ethanol containing 035 mol is cooled from 1250 c C to 620 o C.
There are 2 atoms of carbon in 1 molecule of acetic acid. --- There are 140g of nitrogen in every 460g of NO2. In general which of the following statements correctly describes the reactivity of alkenes.
The molar mass of a molecular substance is the mass per mole of its molecules. This observation is summarized by the law of definite _____. The periodic table can be used to calculate the molar mass of any substance.
Check all that apply. Molar mass is the sum of the weight of all the atoms in the molecule. -There are 1600 amu of oxygen per molecule of acetic acid.
-There are 4 moles of hydrogen atoms per mol of acetic acid. Which of the following statements correctly describe steps used in determining which reactant in a given reaction is limiting. The mole relates the atomic mass of an element expressed in amu with the mass of 1 mole of that element expressed in.
Knowing this we can find A B C. Mass proportions Which of the following statements correctly describe molar mass-The molar mass is the mass in grams per mole of the substance-The periodic table can be used to calculate the molar mass of any substance In a given chemical compound the component _____ are present in _____. Check all that apply Calculate the molar masses of any reactants for which a mass has been given.
Molar masses are expressed in gmol. O 2664. The periodic table can be used to calculate the molar mass of any substance.
Which of the following statements correctly describe CHCOOH acetic acid. The molar mass of a substance equals the number of moles in one gram of the substance. 2108 gmol32 gmol248 gmol.
Use the chemical equation to find the number of moles of the product. Which of the following statements correctly describe CH3COOH acetic acid. 248 gmol002 mol496 g 4.
Select all that apply. Atomic mass of oxygen 160g. Select all that apply.
Since neon has an atomic mass of 2018. Molar mass1211 g00275 mol440 gmol Since N has a molar mass of 14 gmol and O has a molar mass of 16 gmol the formula N2O would produce the correct molar mass. Molar mass of NO2 460g.
Check all that apply. Report the molar mass of nitrogen as 2802gmol. Which of the following statements correctly describes a relationship between empirical formula and molecular formula.
An aqueous solution of KCl molar mass7455 gmol has a molality of 025 molkg and a density of 105 gmL. X The molecular formula is twice the empirical formula 90083003 - 3. The mass of 1 mole of oxygen atoms is therefore equal to g to 4 significant figures.
--- The mass ratio of nitrogen is NO2 is equal to 304. Its molar mass is 2018 gmol. The term molar mass applies to compounds but not to elements.
How many milliliters of 57 M H2SO4 are required to react with 069 g of CuO according to the equati. A The empirical formula molar mass is greater than the molecular formula for all compounds. Complete the unit conversion by entering the correct numbers A B C.
The molar mass of acetic acid is 2902 gmol. K and it s molar mass is 7412 g mol. Molar mass is the sum of the number of all atoms in one molecule.
Which of the following expressions correctly represent the percent by mass of a solute in a solution. Which of the following statements correctly describe molar mass. Click card to see definition.
Select all that apply. B The empirical formula and the molecular formula are the same for all compounds. Tap card to see definition.
Calculate the amount of product that could be formed from each reactant The reactant that produces. This means that 1 mole of oxygen atoms has a mass of 1600 g. This means that 1 mole of oxygen atoms has a mass of 1600 g.
For this problem you will need to know your unit conversions. S for liquid diethyl ether 172 J mol. The molar mass of oxygen is 1600 g.
The molecular formula is. There are 3 ft in 1 yard. Select all that apply.
NPVRT0987 atm0677 L008206 LatmKmol296 K00275 mol Now divide g by mol to get the molar mass. Molar mass is molecular mass. Alkenes react as electrophiles whereas alkanes react as nucleophiles.
You dont need atomic numbers when calculate molar mass. Multiply the atomic mass of nitrogen by 2 in order to calculate its molecular mass 3. Identify that nitrogen exists as N2 and find the atomic mass of nitrogen on the periodic table 2.
There are 4 moles of hydrogen atoms per mole of acetic acid. Do you know the answer. The molar mass of a molecular substance is the mass per mole of its molecules.
The molar mass of oxygen is 1600 gmol. C The empirical formula molar mass is less than or. Divide the mass of the reactant by its molar mass to find the number of moles of the reactant.
An alkene is an electron-rich molecule and therefore can react as a nucleophile. What conversion factors are required to carry out the following concentration unit conversions. -There are 2 atoms of carbon in one molecule of acetic acid -The molar mass of acetic acid is 2902 gmol.
Molar masses are expressed in gmol. The mass of oxygen atoms in a mole of acetic acid is 3200 g. Use the periodic table given in this book to answer this question correctly.
The molar mass of a substance equals the number of moles in one gram of the substance. Molar mass of CuSO45H2O2495gmol Volume of solution 1000 mL Molarity of solution 0200M Mass. Since neon has an atomic mass of 2018.
Q 013 k J The sample contains 337 x 10 2 mol of diethyl ether. Its molar mass is. --- The percentage oxygen in NO2 is equal to 320460 X100.
O 5329. Multiply the number of moles of the product by its molar mass to find the mass of the product. Alkenes are generally unreactive compared alkanes.
Which of the following statements correctly describes a compound with a molar mass of 9008 gmol and an empirical formula of CHO.
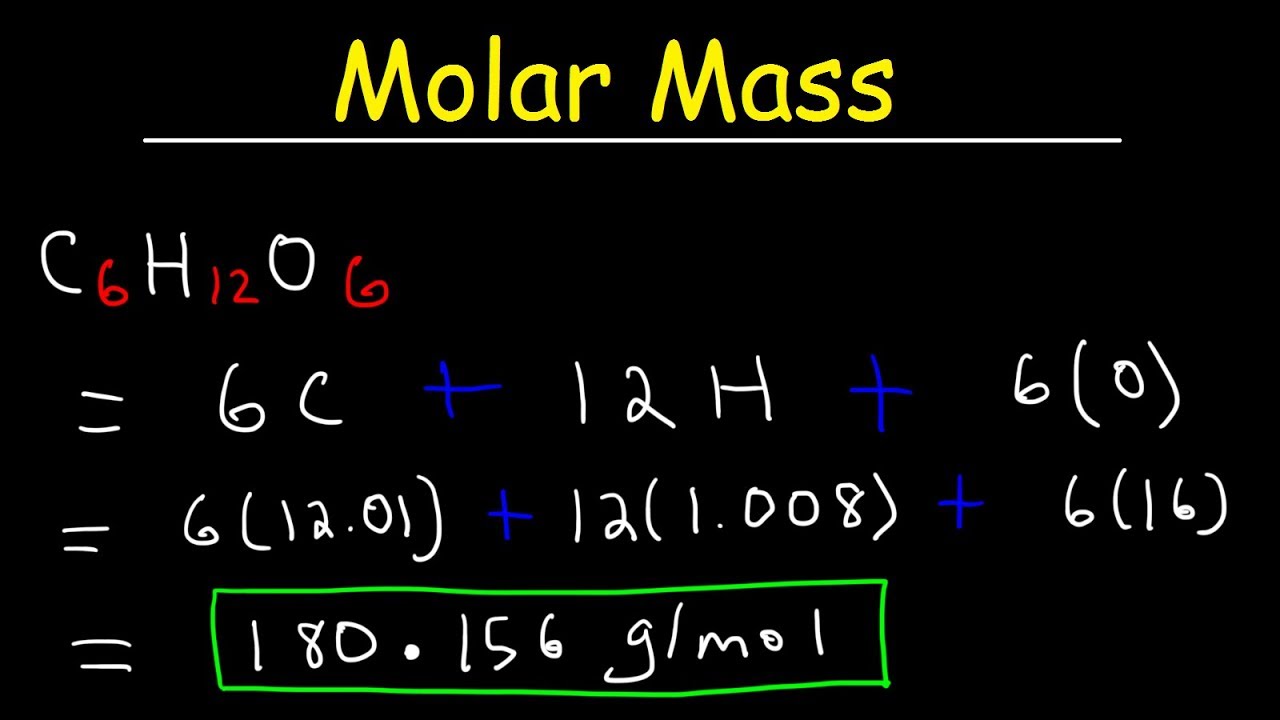
How To Calculate The Molar Mass Of A Compound Quick Easy Youtube

Molar Mass Formula Definition Formula Solved Examples

Mole Conversion Practice Worksheet The Best Worksheets Image Calculating Work Practices Worksheets Worksheets

How To Convert Between Moles Mass Number Of Particles And Volume Of A Gas Mole Day Chemistry Basics Mole

Molar Mass Of The Elements Of The Example Download Table

Molar Mass Molecular Weight Of Feso4 Iron Ii Sulfate Youtube

Molar Mass Molecular Weight Of Al2 So4 3 Aluminum Sulfate Youtube
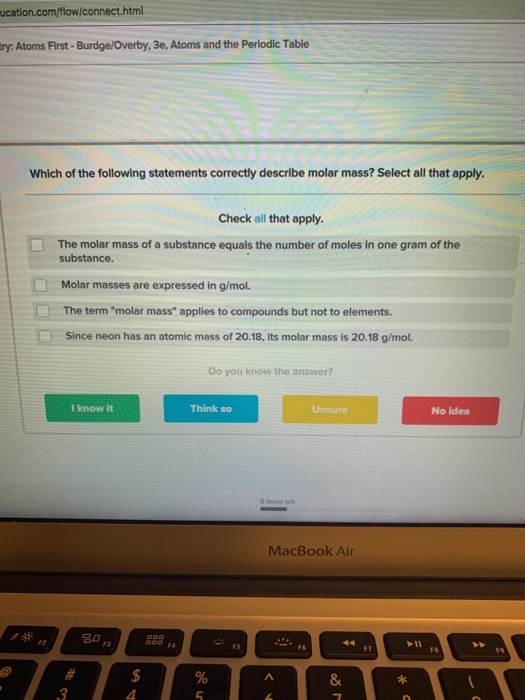
Solved Ucation Com Flow Connect Html Try Atoms First Chegg Com
Difference Between Molar Mass And Molecular Weight Definition Formula Units Calculation

Molar Mass Of H2o2 Hydrogen Peroxide Youtube
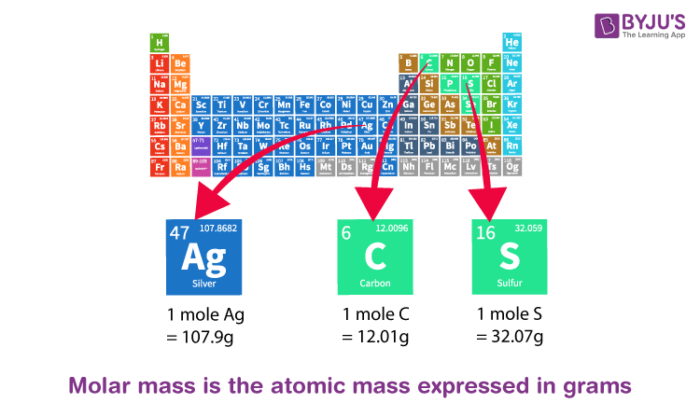
What Is Atomic Mass List Of Elements Sorted By Atomic Mass Of Iron Sulphur Potassium Chlorine Etc

Molar Mass Definition Formula Mole Atomic Mass Chemistrygod

Molar Mass Molecular Weight Definition Formula Examples Of Molar Mass

Calculating Molar Mass And Number Of Moles Worked Example Video Khan Academy

List Of Compounds With Molecular Formula Molar Mass And Retention Download Table

Which Of The Following Phrases Best Describes Process Focus In 2022 Process The Selection Focus
Molar Mass Molecular Weight Of Nh4 2co3 Ammonium Carbonate Youtube


Comments
Post a Comment